Can I Take Zinc With Vitamin D
Vitamin D: A rapid review of the evidence for treatment or prevention in COVID-19
May 1, 2020

Joseph Lee, Oliver van Hecke, Nia Roberts
On behalf of the Oxford COVID-19 Evidence Service Team
Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences
University of Oxford
Correspondence to joseph.lee@phc.ox.ac.uk
VERDICT
We found no clinical evidence on vitamin D in COVID-19. There was no evidence related to vitamin D deficiency predisposing to COVID-19, nor were there studies of supplementation for preventing or treating COVID-19 (Search date upto 4th of April 2020, clinicaltrials.gov searched upto on 23rd April).There is some evidence that daily vitamin D3 supplementation over weeks to months may prevent other acute respiratory infections, particularly in people with low or very low vitamin D status. This evidence has limitations, including heterogeneity in study populations, interventions, and definitions of respiratory infections that include upper and lower respiratory tract involvement.
The current advice is that the whole population of the UK should take vitamin D supplements to prevent vitamin D deficiency. This advice applies irrespective of any possible link with respiratory infection.
Clinicians should treat patients with vitamin D deficiency irrespective of any link with respiratory infection.
Policymakers should attend to public health measures to ensure the population has adequate vitamin D intake.
BACKGROUND
 Vitamin D
Vitamin D
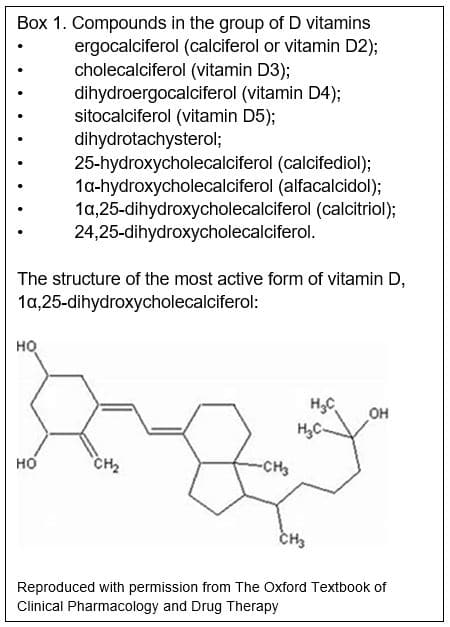
Vitamin D is the name given to a group of vitamins (see box 1). Vitamin D2 (ergocalciferol, found in plants) and vitamin D3 (cholecalciferol, found in animal tissues) are pharmacologically inactive and we convert them to active compounds by hydroxylation in the liver and then kidneys (Figure 1).[1] The liver converts vitamin D3 to 25-hydroxycholecalciferol, which is weakly active. The kidneys then convert this to either 24,25-dihydroxycholecalciferol, which is also weakly active, or to 1α,25-dihydroxycholecalciferol, the most active form of vitamin D (Box 1).[1] Vitamin D is important for bone metabolism, and regulates calcium concentrations in the blood. Vitamin D increases absorption of calcium from the gut and reduces the amount lost by the kidneys. Many tissues have vitamin D receptors, and vitamin D may have other roles.[2]
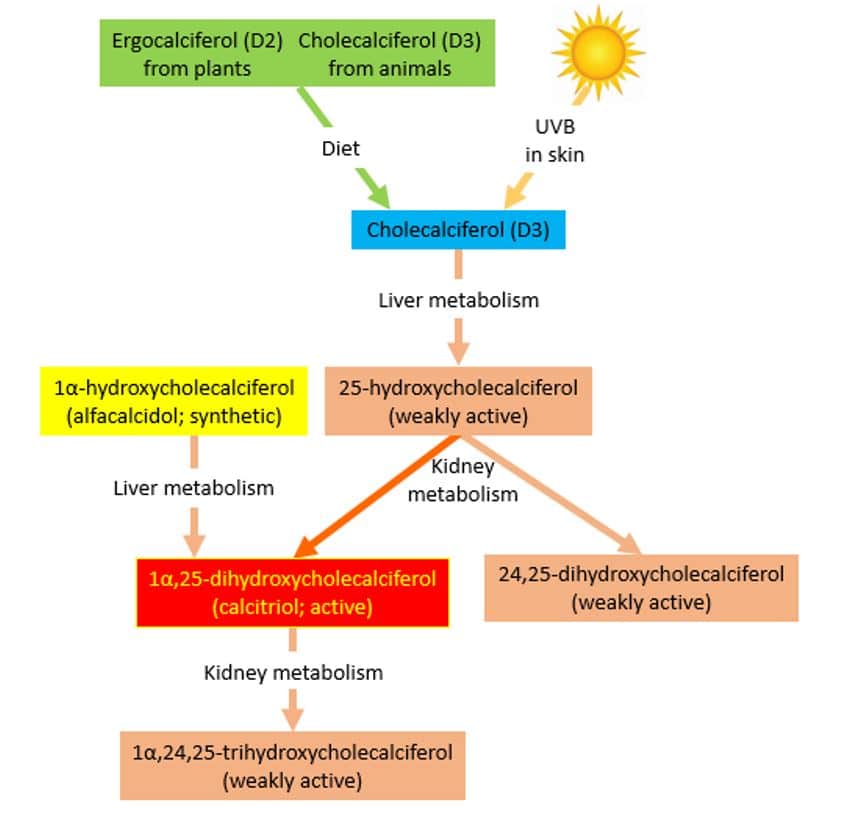
Figure 1: Metabolic pathways of vitamin D, reproduced with permission from The Oxford Textbook of Clinical Pharmacology and Drug Therapy
Sources of vitamin D
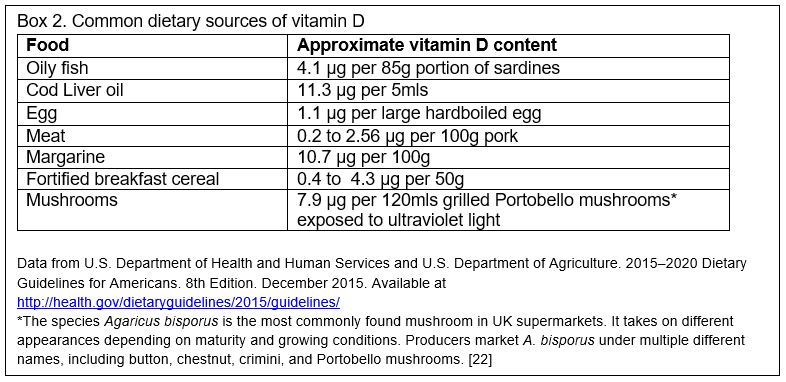
We get vitamin D predominantly by synthesizing D3 in our skin using ultraviolet B (UVB) light, with small quantities obtained from food sources. In countries at high latitudes, the UVB in winter isn't sufficient to synthesize enough vitamin D, so oral intake becomes more important. Dietary sources include animal products like oily fish, red meat, liver, and egg yolks, and fortified foods like infant formula milk, breakfast cereals and margarines (Box 2). Some mushrooms can provide vitamin D if grown under ultraviolet light.[3] The UK government's Scientific Advisory Committee on Nutrition (SACN) advises a daily intake of 10 µg (micrograms) (400 International Units/day)* for everyone over the age of four years living in the UK in order to ensure musculoskeletal health.[4] They estimate that this will meet the needs of 97.5% of the population, but note that it is difficult to achieve this intake with diet alone and recommend supplements.[4] In adults the standard dose for prevention of vitamin D deficiency is 10 µg (400 IU) daily.[5] For young children SACN defined safe intake levels rather than reference intake values; for those less than one year of age 8.5 to 10 µg (340 to 400 IU) per day, and for children aged one to four 10 µg (400 IU) daily.[4]
Vitamin D deficiency
Vitamin D is usually measured in blood serum in the relatively stable 25-hydroxycholecaliferol form (25(OH)D3). In the UK, 25 nmol/L (10ng/ml) is used as the cut off for deficiency, but there is no universally agreed definition so clinical studies have used different values to define vitamin D deficiency.[4]
Vitamin D deficiency is very common, particularly in winter. In January to March in the UK, 30% of people aged 65 years and over and 40% of people aged 19–64 years had serum vitamin D concentrations below 25 nmol/L.[6]
The UK government's SACN report identifies people at high risk of deficiency [4]:
- Infants and children aged under four years old;
- Pregnant and breastfeeding women, particularly teenagers and young women;
- People over 65;
- People who have low or no exposure to the sun, for example those who cover their skin for cultural reasons and those who are housebound or confined indoors for long periods;
- People with darker skin, for example people of African, African-Caribbean, or South Asian family origin.
In vitro evidence for vitamin D's role in immunity and infection
There is in vitro evidence that vitamin D is involved in immune cell responses to some viral and bacterial respiratory pathogens. Vitamin D appears to upregulate genes involved in responses in immune cells that are exposed to Streptococcus pneumoniae.[7] There are laboratory studies showing that respiratory epithelial cells can convert vitamin D to its active form, and that vitamin D metabolites increase cytokines involved in immunity in response to respiratory viruses. However, vitamin D metabolites do not seem to prevent viral replication in cell cultures.[2, 8]
Why consider vitamin D in COVID-19?
Some have speculated that people with low serum vitamin D might be at higher risk of infection with COVID-19, or do worse if infected.[9] There is an overlap between groups at high risk of vitamin D deficiency and groups at high risk of severe COVID-19. Examples include people with chronic disease, older age, and people of black and minority ethnic (BAME) heritage. However, infants and children are at risk of vitamin D deficiency, but are not considered high-risk for severe COVID-19.[10]
Media coverage has highlighted the high burden of COVID-19 in BAME populations.[11] On 10 April 2020 the UK's Intensive Care National Audit and Research Centre (ICNARC) reported that 31% (310/993) of patients requiring advanced respiratory support defined their ethnicity in a non-white category, compared with 24% of patients (99/408) receiving basic respiratory support.[12] These data are intriguing, but we need to be cautious about over-interpreting them. These audits did not set out to examine the risk of vitamin D deficiency as a risk factor for COVID-19 infection, and many factors that might explain this apparent association have not been accounted for.
If vitamin D does have a role in preventing or mitigating the effects of COVID-19 infection, supplementation would be a cheap and low risk intervention. We therefore aimed to review the clinical evidence that vitamin D has a role in preventing or treating COVID-19.
CLINICAL EVIDENCE IN COVID-19
We searched PubMed and Google Scholar for studies that included terms for vitamin D and COVID-19. We found no trials of vitamin D in COVID-19 that have reported results. We did find several studies that are registered, but have not yet reported. None seemed to be masked comparisons to placebo.
A trial of vitamin D3 plus zinc versus usual care is planned in an open-label randomised controlled trial (RCT) in adults over the age of 60 years who are 'institutionalized' but asymptomatic.[13] The dose of vitamin D3 is 2000 IU (50 µg) plus 30 mg of zinc gluconate per day for two months. The primary outcome measure is mortality; the incidence of COVID-19 infection is a secondary outcome.
One trial is testing whether a single oral dose of 25,000 IU (625 µg) of vitamin D (form not specified) will improve mortality in patients who are infected with SARS-CoV-2 but do not have severe symptoms, compared with usual care.[14] The rationale draws on veterinary findings that reduced levels of vitamin D in calves were associated with bovine coronavirus infection.
Another RCT will compare single doses of vitamin D3, 50,000 IU to 200,000 IU (1250 Vs 5000 µg) in people with COVID-19 pneumonia who are over 75 years of age, or over 70 with low oxygen saturations; the primary outcome measure is mortality at 14 days.[15]
Single group studies:
A single group open-label study is using a combination of hydroxychloroquine, vitamins C and D (form not specified), and zinc as prophylaxis in healthy healthcare workers who are at risk of COVID-19 through exposure to infected patients.[16] The same investigator has registered another study, that will give all participants hydroxychloroquine, vitamins C and D (form not specified), and zinc plus azithromycin, with the aim of determining if this combination can effectively treat COVID-19.[17] It is unclear how these studies will meet their stated aims without comparison groups.
VITAMIN D TO PREVENT OTHER ACUTE RESPIRATORY TRACT INFECTIONS
As our searches returned no relevant results, we widened the criteria to include other acute respiratory tract infections (ARTI). ARTIs were defined by most of the studies as a broad group comprising both upper and lower respiratory infections.
Observational evidence suggests that low, particularly very low serum vitamin D concentrations are associated with a higher incidence of ARTIs. A recent meta-analysis of observational studies showed a significantly greater risk of ARTI in patients with the lowest 25(OH)D3 categories compared with the highest categories (odds ratio (OR) 1.83; 95% CI 1.42 to 2.37; I2 = 78.8%,14 studies, n=78,217 adults).[18] They pooled vitamin D categories as defined by the included studies (which varied a great deal), and included diverse ARTIs from upper respiratory tract infections to pneumonia. They looked for a dose response relationship and found a non-linear association between infections and serum vitamin D (highest risk in <37.5nmol/L but risk detectable at <60nmol/L in data from 5 studies of 37,902 participants). Patients were at higher risk of a composite outcome of severe infection or dying in the lowest vitamin D categories compared with the highest (OR 2.46, 95% CI 1.65 to 3.66; I2 = 49.8, 5 studies, n=1495) and also at higher risk of dying (OR 2.46; 95% CI 1.65 to3.66; I2 = 49.8%; 4 studies, n=1422). The authors raised some important concerns about the data, including evidence of publication bias and a high degree of heterogeneity in the results.
Reviews and meta-analyses of RCTs have found a protective effect of vitamin D3 supplementation taken over weeks to months. This was more pronounced in patients with the lowest baseline concentrations of vitamin D (though definitions of deficiency varied).[19, 20] One review of RCTs showed that vitamin D supplementation was associated with reduced ARTIs (OR for combined upper and lower RTI 0.64; 95% CI 0.49 to 0.84; p = 0.0014, I2 = 72%; 11 studies, n = 5389, NNTs over 3 months 9 to 33).[19] They also examined whether other factors might explain this effect, such as infrequent versus daily dosing of vitamin D3, baseline vitamin D status, adults versus children, sex, or healthy participants versus patients. The only significant association was the dosing interval: when vitamin D3 was administered daily it was associated with a significant reduction in ARTIs (OR, 0.51; 95% CI, 0.39 to 0.67), while vitamin D3 had no effect when administered in large doses once per month or less often (OR 0.86; 95% CI, 0.62 to 1.20).
A large meta-analysis of individual patient data from RCTs showed similar findings.[20] Vitamin D3 supplementation resulted in a significant reduction in the proportion of patients experiencing at least one ARTI (adjusted OR 0.88, 95% CI 0.81 to 0.96, P=0.003; NNT=33 [20 to 101], n=10,933). The effect of supplementation was greatest in patients with serum concentrations below 25 nmol/L (adjusted OR 0.58, 95% CI 0.40 to 0.82, NNT = 8 [5 to 21], n=538). Daily or weekly dosing seemed to be protective, even when baseline concentrations were above 25 nmol/L, but intermittent larger doses were not effective, even in patients with vitamin D deficiency. Subgroup analyses did not show significant effects when upper and lower respiratory tract infections were analysed separately.
Lastly, an overview of systematic reviews of vitamin D for all non-skeletal conditions suggests that vitamin D2 or D3 supplementation has no important clinical effect on most conditions, including chronic inflammation, strengthening the hypothesis that low vitamin D status is a consequence of ill health, rather than its cause.[21]
CONCLUSIONS
We found no clinical evidence that vitamin D supplements are beneficial in preventing or treating COVID-19. We would need evidence from well-masked randomized trials to determine if there are effects, before recommending vitamin D3 supplements for treating or preventing COVID-19 infection.
There is some evidence that vitamin D may have a role in preventing other respiratory infections, particularly for people with low or very low vitamin D status. Whilst this evidence comes from systematic reviews of randomised trials, it has many limitations, including heterogeneous definitions of respiratory infections, study populations, interventions and definitions of vitamin D deficiency.
People at risk of vitamin D deficiency should in any case take supplements in line with current guidance. Currently the whole UK population is advised to take supplements. Similarly, clinicians should continue to treat people with vitamin D deficiency – but not because of any possible association with respiratory infection. Policymakers should attend to the recommendations of the SACN, including developing food-based strategies for the general population to achieve adequate vitamin D intake.
Disclaimer: This article has not been peer-reviewed; it should not replace individual clinical judgement and the sources cited should be checked. The views expressed in this commentary represent the views of the authors and not necessarily those of the host institution, the NHS, the NIHR, or the Department of Health. The views are not a substitute for professional medical advice.
Acknowledgements: We would like to thank Professor Susan Jebb and Dr Jeffrey Aronson for their helpful comments, and Dr Aronson for permission to reproduce material from The Oxford Textbook of Clinical Pharmacology and Drug Therapy.
AUTHORS
Joseph Jonathan Lee is a General Practitioner and doctoral researcher based at the Nuffield Department of Primary Care Health Sciences, University of Oxford.
Oliver van Hecke is a General Practitioner and NIHR Academic Clinical Lecturer based at the Nuffield Department of Primary Care Health Sciences, University of Oxford.
Nia Wyn Roberts is an Information Specialist based at the Nuffield Department of Primary Care Health Sciences, University of Oxford.
*1 International Unit (IU) is the biological equivalent of 25 nanograms of cholecalciferol or ergocalciferol; i.e. 40 IU = 1 microgram of vitamin D. It is defined as the total amount of vitamin D that will produce a narrow line of calcium deposit in the rachitic metaphyses of the distal ends of the radii and ulnae of standard rachitic rats within 10 days.[1]
SEARCH TERMS
We searched Pubmed, Google Scholar and medRxiv on 4 April 2020 for evidence for a number of reviews of substances in COVID-19, including vitamin D. For vitamin D we included the search terms: Vitamin D* OR vit d* OR colecalciferol, AND coronavirus, SARS-Cov-2, 2019-NCov, and COVID-19; and broader search terms Vitamin D* OR vit d* OR colecalciferol AND bronchitis OR common cold OR influenza OR pneumonia OR SARS OR MERS OR severe respiratory infections OR acute respiratory infections OR "Respiratory Tract Infections"[Mesh]. We also searched clinicaltrials.gov for registered trials of vitamin D in COVID-19 on 23/04/2020. In addition, we included reviews of the existing literature on this topic. We provide a narrative summary of the current literature.
REFERENCES
- Grahame-Smith DG, Aronson JK. The Oxford Textbook of Clinical Pharmacology and Drug Therapy. Oxford: Oxford University Press; 2002.
- Hansdottir S, Monick M, Hinde S, Lovan N, Look D, Hunninglake G. Respiratory Epithelial Cells Convert Inactive Vitamin D to its Active Form. J. Immunol. 2008; 181: 7090–7099.
- Cardwell G, Bornman JF, James AP, Black LJ. A review of mushrooms as a potential source of dietary vitamin D. Nutrients 2018; 10: 1–11.
- Public Health England. Vitamin D and Health 2016 [Internet]. Sci. Advis. Comm. Nutr. 2016 Available from: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report.
- Joint Formulary Comittee. British National Formulary [Internet]. London: BMJ Group and Pharmaceutical Press; 2019.Available from: https://bnf.nice.org.uk/drug/colecalciferol.html#indicationsAndDoses.
- Bates B, Lennox A, Prentice A, Bates C, Page P, Nicholson S, Swan G. National Diet and Nutrition Survey Results from Years 1, 2, 3 and 4 (combined) of the Rolling Programme. London; 2008.
- Olliver M, Spelmink L, Hiew J, Meyer-Hoffert U, Henriques-Normark B, Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J. Infect. Dis. 2013; 208: 1474–1481.
- Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015; 7: 4240–4270.
- Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020; 12: 988.
- Coronavirus (COVID-19): Shielded patients list [Internet]. Available from: https://digital.nhs.uk/coronavirus/shielded-patient-list.
- Croxford R (BBC). Coronavirus: Ethnic minorities "are a third" of patients [Internet]. 2020.Available from: https://www.bbc.co.uk/news/uk-52255863.
- ICNARC. ICNARC report on COVID-19 in critical care. 2020; : 1–9.
- Seguy D. Impact of Zinc and Vitamin D3 Supplementation on the Survival of Aged Patients Infected With COVID-19 (ZnD3-CoVici) [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT04351490.
- Castillo MJ. Vitamin D on Prevention and Treatment of COVID-19 (COVITD-19) [Internet]. NCT04334005 2020.Available from: https://clinicaltrials.gov/ct2/show/NCT04334005.
- Annweiler C. COvid-19 and Vitamin D Supplementation: a Multicenter Randomized Controlled Trial of High Dose Versus Standard Dose Vitamin D3 in High-risk COVID-19 Patients (CoVitTrial) [Internet]. 2020.Available from: https://clinicaltrials.gov/ct2/show/record/NCT04344041.
- Haza S. A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection (HELPCOVID-19) [Internet]. 2020.Available from: https://clinicaltrials.gov/ct2/show/record/NCT04335084.
- Haza S. A Study of Quintuple Therapy to Treat COVID-19 Infection (HAZDpaC) [Internet]. 2020.Available from: https://clinicaltrials.gov/ct2/show/NCT04334512.
- Pham H, Rahman A, Majidi A, Waterhouse M, Neale RE. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2019; 16.
- Bergman P, Lindh ÅU, Björkhem-Bergman L, Lindh JD. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One 2013; 8.
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Stelmach I, Kumar GT, Urashima M, Camargo CA. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017; 356.
- Autier P, Mullie P, Macacu A, Dragomir M, Boniol M, Coppens K, Pizot C, Boniol M. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017; 5: 986–1004.
- Stamets P. Growing Gourmet and Medicinal Mushrooms. 3rd ed. Berkely, California: Ten Speed Press; 2000.
FURTHER REVIEWS:
NICE published a rapid review of the evidence for Vitamin D for COVID-19 on the 29th of June 2020. It can be found here.
SACN published an update to their review of vitamin D and acute respiratory tract infections on 11th June 2020. It can be found here.
Can I Take Zinc With Vitamin D
Source: https://www.cebm.net/covid-19/vitamin-d-a-rapid-review-of-the-evidence-for-treatment-or-prevention-in-covid-19/
